Cyclopropanes
Cyclopropanes are a family of organic compounds containing the cyclopropyl group. The parent is cyclopropane (C3H6).
Synthesis and reactions
[edit]Most cyclopropanes are not prepared from the parent cyclopropane, which is somewhat inert. Cyclopropyl groups are often prepared by cyclization of 1,3-difunctional alkanes. An example of the former, cyclopropyl cyanide is prepared by the reaction of 4-chlorobutyronitrile with a strong base.[1] Phenylcyclopropane is produced analogously from the 1,3-dibromide.[2]
A second major route to cyclopropanes entails addition of methylene (or its substituted derivatives) to an alkene, a process called cyclopropanation.[3]
Substituted cyclopropanes undergo the reactions associated with the cyclopropyl ring or the substituents. Vinylcyclopropanes are a special case as they undergo vinylcyclopropane rearrangement.
Applications and occurrence
[edit]
Cyclopropane derivatives are numerous.[4] Many biomolecules and pharmaceutical drugs feature the cyclopropane ring. Famous example is aminocyclopropane carboxylic acid, which is the precursor to ethylene, a plant hormone.[5]
The pyrethroids are the basis of many insecticides.[6] Several cyclopropane fatty acids are known.
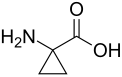
1-Aminocyclopropane-1-carboxylic acid plays an important role in the biosynthesis of the plant hormone ethylene.[5]
References
[edit]- ^ Schlatter, M. J. (1943). "Cyclopropyl Cyanide". Organic Syntheses. 23: 20. doi:10.15227/orgsyn.023.0020.
- ^ "Cyclopropylbenzene". Organic Syntheses. 44: 30. 1964. doi:10.15227/orgsyn.044.0030.
- ^ Maruoka, Keiji; Sakane, Soichi; Yamamoto, Hisashi (1989). "Selective Cyclopropanation of (S)-(−)-Perillyl Alcohol: 1-Hydroxymethyl-4-(1-Methylcyclopropyl)-1-Cyclohexene". Organic Syntheses. 67: 176. doi:10.15227/orgsyn.067.0176.
- ^ Rappoport, Zvi, ed. (1995). The Chemistry of the Cyclopropyl Group. The Chemistry of Functional Groups. Vol. 2. doi:10.1002/0470023481. ISBN 0471940747.
- ^ a b Kieber, Joseph J.; Schaller, G. Eric (2019-07-01). "Behind the Screen: How a Simple Seedling Response Helped Unravel Ethylene Signaling in Plants". The Plant Cell. 31 (7): 1402–1403. doi:10.1105/tpc.19.00342. ISSN 1040-4651. PMC 6635871. PMID 31068448.
- ^ Faust, Rüdiger (2001). "Fascinating Natural and Artificial Cyclopropane Architectures". Angewandte Chemie International Edition. 40 (12): 2251–2253. doi:10.1002/1521-3773(20010618)40:12<2251::AID-ANIE2251>3.0.CO;2-R. PMID 11433485.

